Determination of Sodium by Flame Emission Spectroscopy
Flame photometry is also named as flame emission spectroscopy because of use of flame to provide the energy of is a controlled flame test with the intensity of the flame colour. Atomic emission spectroscopy AES employing flames also called flame emission spectroscopy FES or flame photometry has found widespread application in elemental.
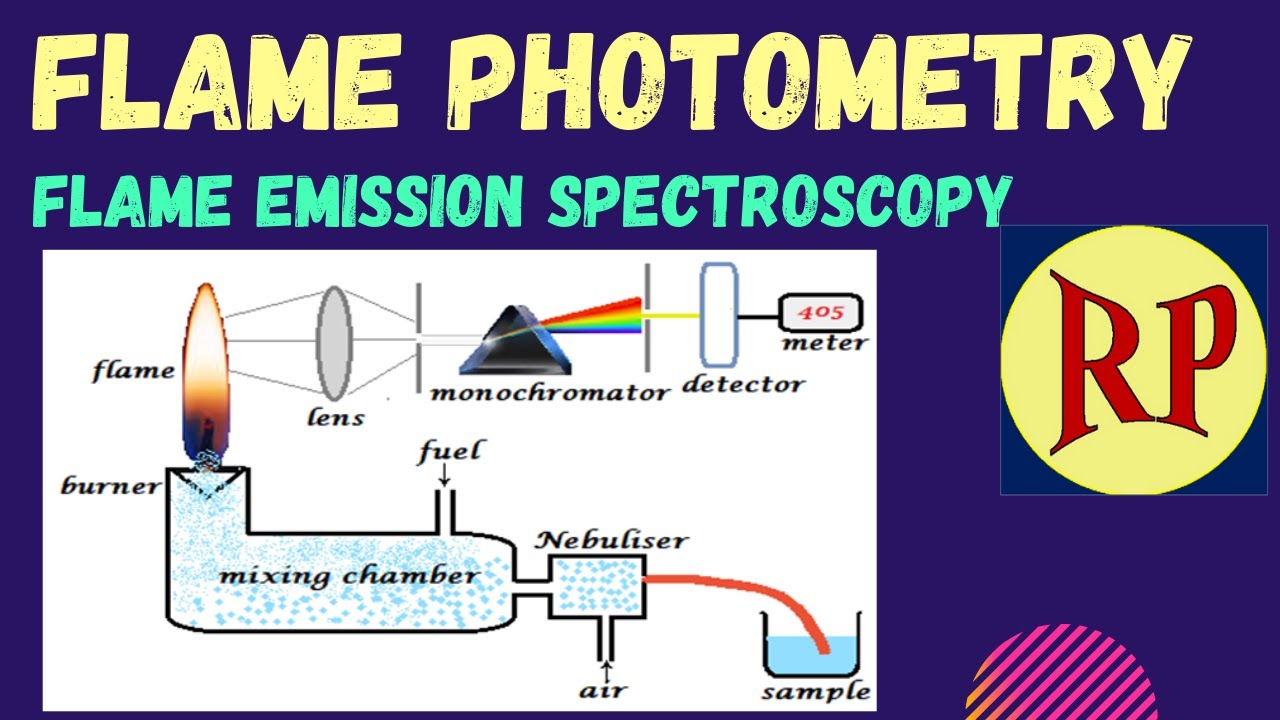
Flame Photometry Flame Emission Spectroscopy Fes Atomic Emission Spectroscopy Aes Youtube
Flame AES also called flame photometry has been an essential technique within clinical laboratories for measuring the major cations sodium and.

. Sodium sample is dissolved by ultrasonic humidifier. Sodium and potassium The major cation of the extracellular fluid is sodium. A analytical method has been developed to determinate potassium in sodium by flame atomic emission spectroscopy.
The purpose of this lab is to determine if the amount of sodium in the tonic water corresponds to the amount of benzoate in tonic water and to compare the amount of sodium to the amount. These metals are easily excited in flames and consequently can be. As specifications for trace metals are lowered in semiconductor chemicals analysis time and the potential for contamination have increased.
Because of the very narrow ca. Na Determination and Self-Absorbance Refer to procedure 413 on page 50 in the Varian AA-12751475 Operation Manual for specific directions regarding flame emission operation with. The body requirement is for 1-2 mmol per.
As an analytical method atomic emission is a fast simple and sensitive method for the determination of trace metal ions in solution. Experimental procedures and typical student dataresults are provided for an experiment determining the sodium parts per million in salt substitute with an error of. Flame photometry is also known as atomic emission spectrometry.
Determination of Sodium Potassium Magnesium And Iron Oxides In Portland Cement By Flame Photometry Research Laboratory Office of Testing and Research Report No. Atomic emission spectroscopy AES is a convenient method for the determination of alkali metals in water samples. Sodium is used as a coolant in China experiment fast reactor CEFR.
Potassium in sodium has an influence on heat property of reactor. A analytical method has been developed to determinate. INSTRUMENTATION Buck Scientific Flame Photometer Model PFP7 The PFP7 Flame Photometer is a low-temperature airnatural gas flame atomic emission photometer designed for the.
When a solution containing cations of sodium and potassium is spayed into flame the solvent evaporates and ions are. The instrument readings R for these solutions were 312I 5409. The typical daily diet contains 130-280 mmol 8-15 g sodium chloride.
Na and K are typically. Flame Atomic Emission Spectroscopy. The sodium in a series of cement samples was determined by flame emission spectroscopy.
The flame photometer was calibrated with a series of NaCl standards that contained sodium equivalent to 0200400600 and 800 pg Na20 per ml.
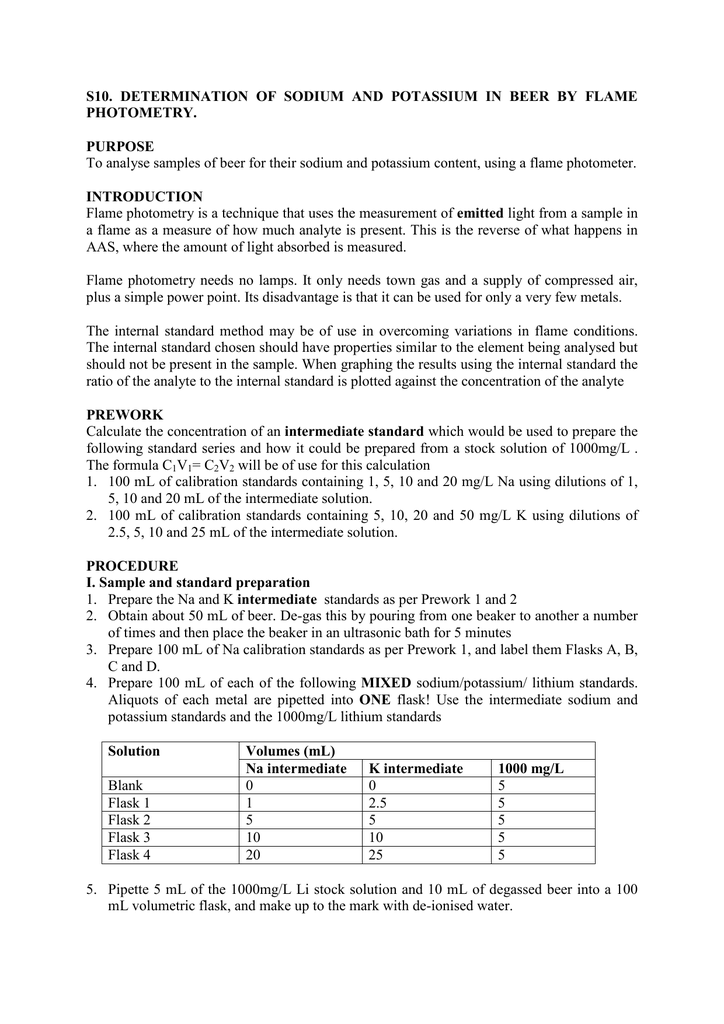
S10 Determination Of Sodium And Photometry Purpose

Emission Spectra And Photographs From The Flame Tests Of Lithium Download Scientific Diagram

Analysis Of Flame Emission Spectroscopy And Atomic Absorption Download Scientific Diagram

No comments for "Determination of Sodium by Flame Emission Spectroscopy"
Post a Comment